Which Measurement Has the Greatest Number of Significant Figures
They all had he same number of significant figures so there was none that the greatest number. How many significant figures are in the number 45359.
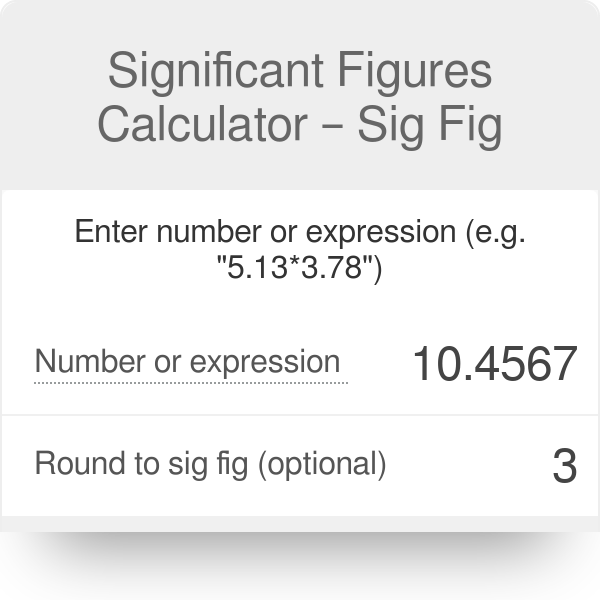
Significant Figures Calculator Sig Fig
Which reading has the greatest number of significant figures.

. Thus the number with the greatest number of significant figures is 6060. 606 has 3 significant figures while 606 has also 3 significant figures. Correct option is C If there is a decimal point the rightmost digit is the last or least significant figure.
6060 has 4 significant figures including the zero after the decimal and the zero between non-zeros. So 008076 is the. Which measurement had the greatest number of significant figures.
The graduated cylinder had the measurement with the most significant figures with 4It had the highest accuracy in measurement and that is reflected in the higher number of significant figures allowing us to measure the most accurate reading possible. 8580 5707572 5708 570757 57076. Correctly rounded the product 2000 cm 200 cm is.
What is the difference between using a 50 mL buret vs. Enter whole numbers real numbers scientific notation or e notation. Rules To Count The Significant Figures.
Find the area of a rectangle 21 cm by 324 cm. A V BI U Ix 12. Hich of the following measurements has the greatest number of significant figures.
These digits provide meaningful information about the precision of a calculation or measurement. 400 x 101 cm2. Give a numerical answer.
Two atoms will always have the same atomic number if they have the same. 0982 cm x 471 cm x 83 cm. Which measurement has the greatest number of sig figs.
What is the correct answer to this calculation reported using the proper number of significant figures. Determine the number of significant figures in each of. Which of the following numbers has least number of significant figures.
Which measurement contains a total of three significant figures. Units and Measurement. Which measurement had the greatest number of significant figures.
The number 12378162 rounded to 4 significant digits is 1238. How many significant figures does it have. Thus the answer to 0024 x 1244 would be rounded off to contain two significant figures since the factor with the lesser number of significant figures 0024 has only two such figures.
Which of the following measurements has the greatest number of significant figures. You can count the number of Significant figures from these rules. Example inputs are 3500 350056 35 x 103 and 35e3.
60600 has 3 significant figures. Hence 1238 is the answer. 8 0 7 6 0.
Which measurement had the greatest number of significant figures. For example 2213 has 4 and 299792458 has 9 significant digits. Zeroes sandwiched anywhere between the non-zero digits are significant.
To use an exact value in the calculator give the value to the greatest number of significant figures in the calculation. In this example you would want to enter 200 for the constant value so that it has the same number of significant figures as the radius entry. Which measurement has the greatest number of significant figures.
All the reported non-zero numbers in a measurement are significant. Fill in the volume column in the table below with the data you recorded. A 50 ml graduated cylinder.
Is the volume of liquid contained in each glassware the same as the measurement you recorded for the reading. Correct answer - 1 point 17. Determine the number of significant digits from the following given numbers.
Measuring Container Volume beaker Erlenmeyer flask graduated cylinder 2 Laboratory Skills_Essay2_Volume Measurements Significant Figures EXPERIMENT 1. 0 8 0 7 6. АА АУ B I U S I 2 Volume Measuring Container 40ml beaker 49mL Erlenmeyer flask 44mL graduated cylinder EXPERIMENT 1.
610348 709 969192 - 1436. 10525 g9162 g9162 g100. The number of significant figures is still determined by the accuracy of the initial speed value in ms - for example 1523 36 5483.
The one had the greatest value however was the first volume estimated in the beaker. Fill in the volume column in the table below with the data you recorded. So for this example you would enter 1523 3600 into the calculator.
8 0 2 0 0. Perform the mathematical operation and report the answer with the correct significant figures. 84 0084 58480 2005 8400.
01 44000 g O2 404 g 03 40449 O 4 040004 g. Correctly rounded 32 x 10-3 cm - 27 x 10-4 cm is. Number of significant figures.
12 108 cm. Calculate the volume in cm3 of a rectangular solid with the given dimensions rounding your answer to the proper number of significant figures. You can use this calculator for significant figures practice.
Test your ability to find how many significant figures are in a number. 1 Laboratory Skills_Essay1_Volume Data from Experiment EXPERIMENT 1. This is the number of significant figures you should have in your answer the product.
Solve the following 476 562 3321 and find the number of significant digitsfigures. Count how many significant figures are in a number and find which digits are significant. The resulting answer would be 470 which has 3 significant figures.

Table With Rules For Significant Figures In Measured Numbers School Hacks Math Algebra

Significant Figures Teaching Chemistry Chemistry Classroom College Chemistry

Identify Significant Digits Worksheets Chemistry Worksheets Teaching Chemistry Persuasive Writing Prompts
Chemistry Lesson Significant Digits Measurements Get Chemistry Help

Significant Figures Notes Chemistrynotes Com Chemistry Notes Teaching Chemistry Chemistry

Accuracy Precision And Significant Figures Physics
Chemistry Lesson Significant Digits Measurements Get Chemistry Help
Rules For Significant Digits Ppt Video Online Download
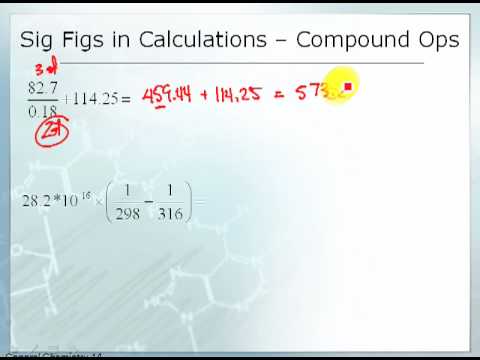
2 4 Significant Figures In Calculations Chemistry Libretexts

1 9 Measurement And Significant Figures Chemistry Libretexts
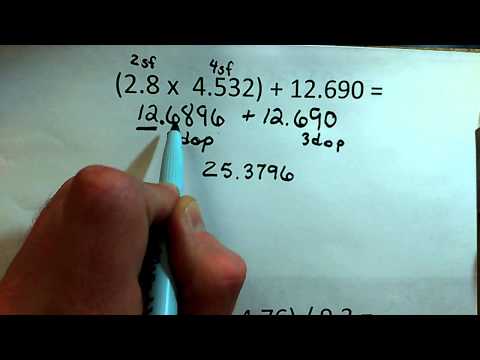
2 4 Significant Figures In Calculations Chemistry Libretexts
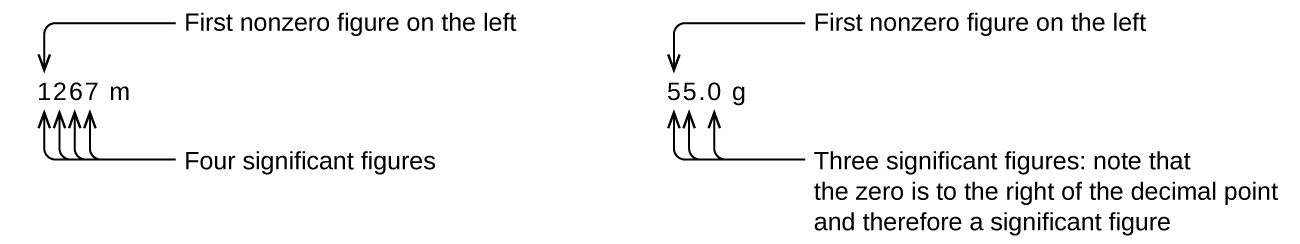
1 5 Measurement Uncertainty Accuracy And Precision Chemistry
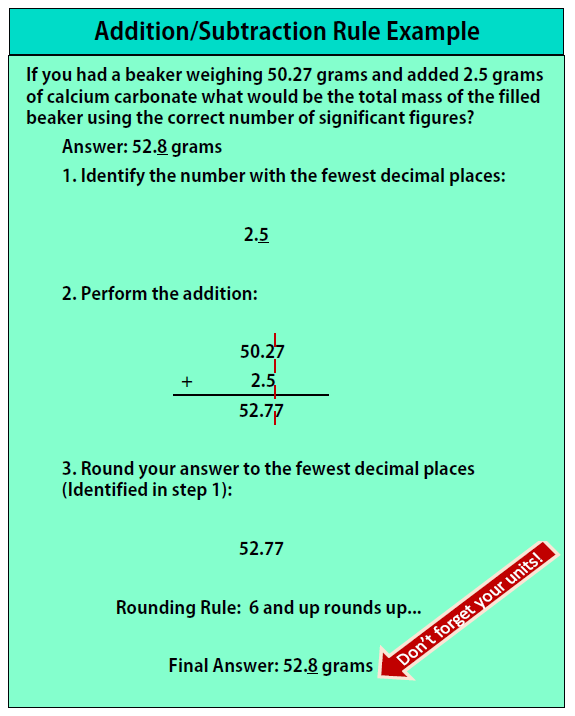
Chapter 1 Measurements In Chemistry Chemistry

Significant Figures Made Easy Youtube
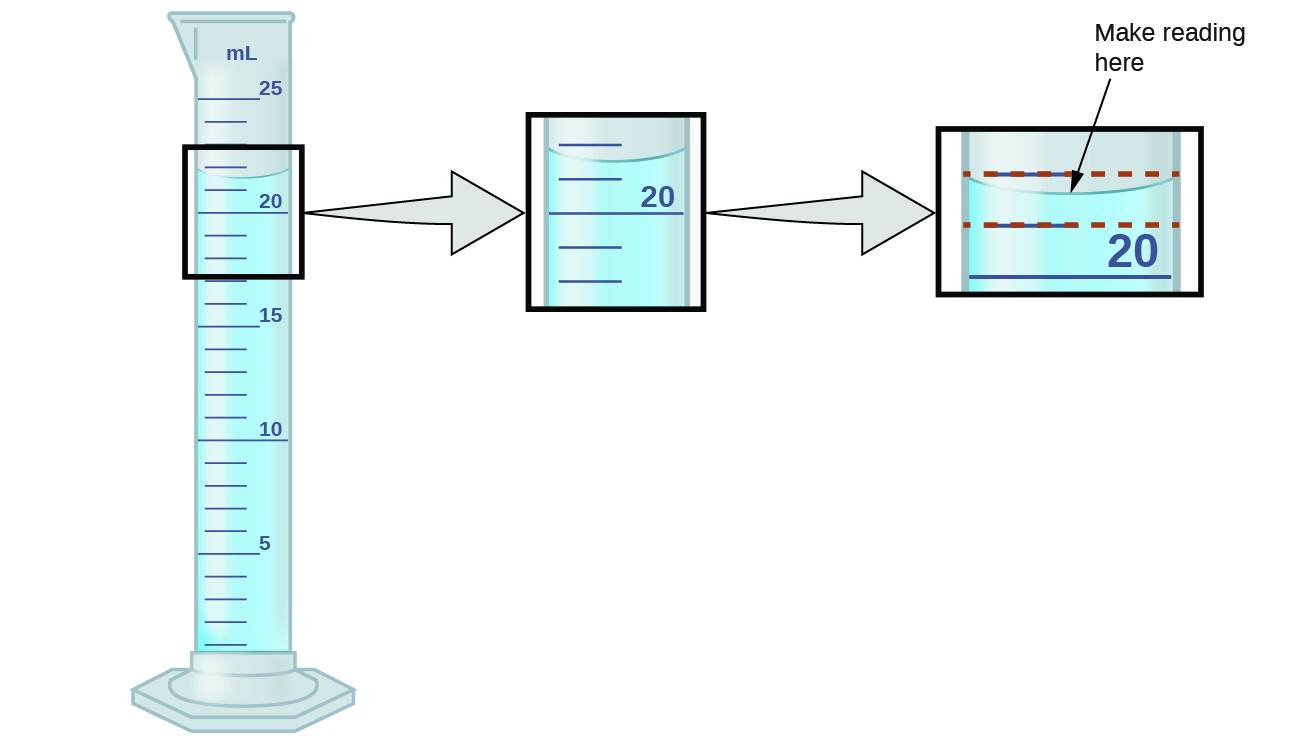
1 5 Measurement Uncertainty Accuracy And Precision Chemistry
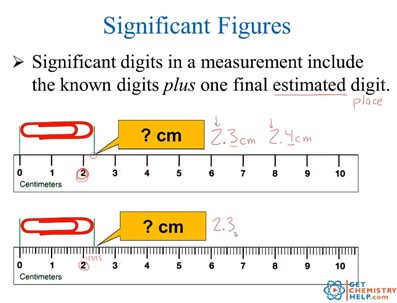
Chemistry Lesson Significant Digits Measurements Get Chemistry Help


Comments
Post a Comment